What Relationship Does Charles Law Describe
The balloon has a fixed volume but there is a hole at the bottom. V i T i V f T f.
Charle S Law Explanation In Terms Of Gaseous State Qs Study
2 See answers Advertisement Advertisement Brainly User Brainly User The relationship between temperature and volume.

. When does the Charles law fail. Upon completing this lesson you will be able to. What relationship does Charless law describe.
Pdf Perpetually Astride Eden S Boundaries The Limits To The Limits Of Law And The Semiotic Inconsistency Of Legal Enclosures The differences are that Boyles Law is a direct relationship while Charles Law is an inverse relationship. Charles Law is a gas law describing how gases expand when heated or the relationship of temperature and the volume of gas. The relationship between temperature and volume.
Not means the volume of a given mass of a gas depends directly on its absolute temperature. This law is valid as long as the pressure and the amount of gas are constant. The pool floats forms yet another real-life example of Charles Law.
V1 T2 T1 V2. It is mainly taught in Physics and Chemistry. So Charles Law is a gas law that relates the temperature and the volume of the gas a constant pressure and a constant number of moles.
The law also states that the Kelvin temperature and the volume will be in direct proportion when the pressure exerted on a sample of a dry gas is held constant. Or we can say we buy T is constant. So the extra volume flows out of the hole in the bottom of the balloon.
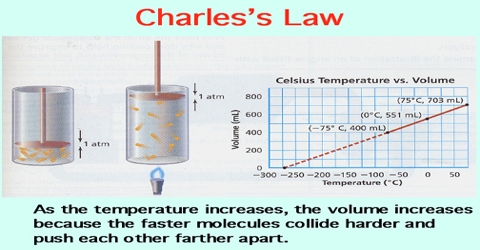
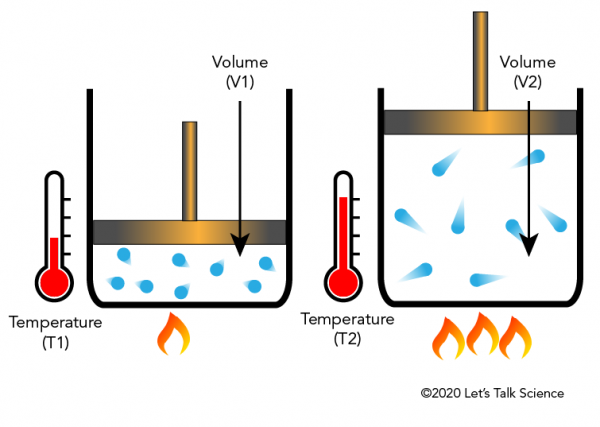
Charles law states that the volume of an ideal gas is directly proportional to the absolute temperature at constant pressure. Charless Law Relationship Between Temperature And Volume Relationship Between Temperature And Volume. V T This means that when the gas in a hot air balloon is heated the gas expands.
The differences are that Boyles Law is a direct relationship while Charles Law is an. Charles Law Formula and Explanation It states that the volume of a fixed mass of a gas is directly proportional to the temperature. See answer 1 Best Answer.
And and so that relationship is to find as C one over the initial temperature. The relationship between temperature and volume. Explain the relationship between.
What relationship does Charles law describe. What happens to a gas when its temperature is increased. It is a quantitative relationship between temperature and volume of a gas.
What does Charles Law describe. What do Boyles law and Charles law have in common. At what celcius temperature does the volume of a gas become zero according to Charles law.
T one is the same as the quotient between the two tea too. Charless law a statement that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature if the pressure remains constant. Also asked which two variables are isolated in Charles Law.
Charless Law states that the Volume V of a gas is directly proportional to the temperature T. V α T when pressure and number of moles are constant V kT. Thus the answer would be A because it talks abt the volume and the temp.
Can I get brainliest You just saved a brothers life Advertisement Advertisement alphabetsoup alphabetsoup Answer. The relationship between temperature and volume. Charless Law In 1787 the French scientist Jacques Charles discovered that volume of a gas varies when we change its.
Question six which asks us to describe Charles is law. Describes direct relationship between gas temperature and its volume. Hello Today well be talking about Chapter 12.
This law applies to ideal gases held at a constant pressure where only the volume and temperature are allowed to change. This law was formulated in the year 1780 by French physicist Jacques Charles. Often the equation V 1 T 1 V 2 T 2 is used to make calculations involving Charles Law.
What relationship does Charles law describe. This is the statement of Charless law or this is the relation between the volume and absolute temperature of guests. What is the relationship between the variables in Charles Law.
What should the graph for charles law look like. Charles Law says that the volume of a gas is directly proportional to the temperature of the gas. Charles Law is a direct relationship between temperature and volume.
However the basketball gains its volume back when the environment is changed ie you bring it in a warm room. Charles Law definition expresses the direct relationship between absolute Kelvin temperature of a given mass of gas and the volume when pressure is kept constant. Charles Law is expressed as.
Is Charles law a direct or. The relationship between temperature and volume B. You might have observed that after you inflate a pool float and push it.
See answer 1 Best Answer. According to this law the volume of the given mass of a gas is directly proportional to the absolute temperature when the pressure is kept constant. It was given by French scientist J.
Is Charles law a direct relationship. Which of the following shows explicitly the relationship between Boyle law and Charles s law. Charles Law is a direct relationship between temperature and volume.

What Is Charles Law In Physics A Plus Topper
Charles Law And Gay Lussac S Law Let S Talk Science

Charles Law With Statement Equation Graphs Examples Chemistrygod

0 Response to "What Relationship Does Charles Law Describe"
Post a Comment